MISSION
To accelerate the transition to a renewable energy society by discovering new materials, chemicals, and processes through multi-scale simulation and data science.
RESEARCH
We interface multi-scale materials simulation and data science. Specifically, we develop innovative methods that accelerate materials design.
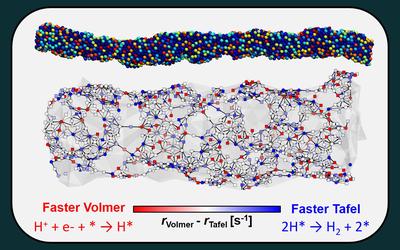
Latest Publications
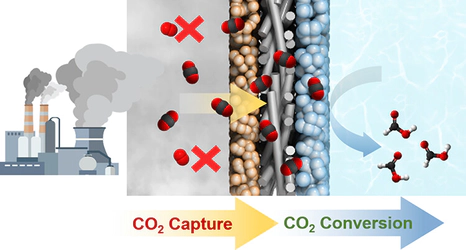
Integrated capture and conversion of low-concentration CO2 is a critical step toward carbon neutrality. Here, we demonstrate a gas diffusion electrode (GDE) modified with oxygen- and nitrogen-functionalized porous graphitic carbon (ONC) that enables efficient electroreduction of dilute CO2. Under 15% CO2 gas (without O2), the ONC-modified GDE achieved a CO2-to-formic acid conversion rate of 250 μmol/h·cm2, 2.5 times higher than that of the bare GDE, with a Faradaic efficiency (FE) of 98%. Even in flue gas containing 8% O2, the modified GDE achieved 22 μmol/h·cm2 formic acid production (8% FE) at −1.4 VRHE. Mechanistic and simulation studies revealed that the oxygen functional groups in ONC enhance CO2 adsorption while suppressing O2 permeation, imparting strong oxygen tolerance. In particular, the ONC-modified GDE remains active at a CO2 concentration as low as 1% and 400 ppm, suggesting potential applicability in integrated capture–conversion systems that utilize dilute CO2 streams from flue gas and ambient air.
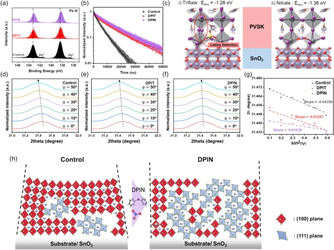
Additive engineering is increasingly being recognized as a critical strategy in the two-step fabrication of perovskite solar cells (PSCs), where the morphology and crystallinity of the initial PbI2 layer strongly influence the subsequent perovskite conversion and interfacial quality. In this study, we addressed the limited understanding of anionic effects in additive engineering by incorporating diphenyliodonium (DPI)-based ionic additives with various counter anions into a PbI2 precursor to control early stage crystallization. The nitrate-based additive (DPIN) promoted the formation of a porous PbI2 framework, enhancing the diffusion of organic halides and enabling complete perovskite conversion. Furthermore, DPIN promoted preferential crystal growth along the (111) facets, particularly in deeper regions of the film, as revealed by grazing-incidence X-ray diffraction (GIXRD). Backside characterization confirmed an improved buried interface morphology with reduced residual PbI2 and fewer pinholes. Density functional theory (DFT) calculations revealed that nitrate ions effectively passivate iodine vacancies at the SnO2/perovskite interface while preserving lattice integrity. These combined effects result in enhanced film quality and device stability. The DPIN-treated device achieved a power conversion efficiency of 25.65 % and retained 95 % of its initial efficiency after 1050 h of ambient storage, along with over 90 % retention under continuous illumination for 500 h, highlighting the dual benefits of nitrate-assisted crystallization and interface engineering.
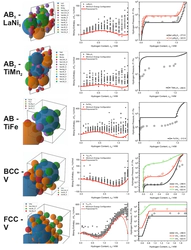
Metal hydrides are promising materials for storing, compressing, and purifying hydrogen by reversibly absorbing and releasing it. Accurate first-principles based prediction of the pressure–composition–temperature (PCT) relationships of metal hydrides can enable predictive optimization of hydrogen capacity and sorption pressures. In this work, we introduce a novel computational framework that integrates density functional theory (DFT) with a Python-based PCT Simulation Toolkit to predict PCT diagrams with high accuracy. By using only structural input data from the metallic phase, this toolkit automates the detection of interstitial voids, generates input files for DFT calculations, and constructs thermodynamic models based on para-equilibrium principles. We validate this approach across four major metal hydride classes– BCC alloys, AB5, AB2, and AB compounds– and demonstrate that even with minimal computational effort, key hydrogen sorption characteristics can be reliably determined. To evaluate the influence of exchange and correlation, we tested three different functionals– PBE, PW91, and r2SCAN– while applying the quasi-harmonic approximation to incorporate vibrational free energy contributions. Our results show that hydrogen capacity predictions achieve a mean accuracy of 95%, while sorption pressures are modeled within one order of magnitude of experimental values. Furthermore, we successfully extend this methodology to the BCC NbTiV multicomponent system, demonstrating its capability to construct comprehensive thermodynamic databases. This framework enables rapid and accurate exploration of known metal hydrides to design optimized alloys for new applications. Additionally, it serves as a predictive tool for designing novel hydrogen storage materials.
Latest News and Gallery



