Elucidating Conductivity and Nitriding Resiliency in (Al/H)-ZnO Coatings for NH3-Fueled SOFC Separators via SEM-EDX and MLP-DFT Protocols
Abstract
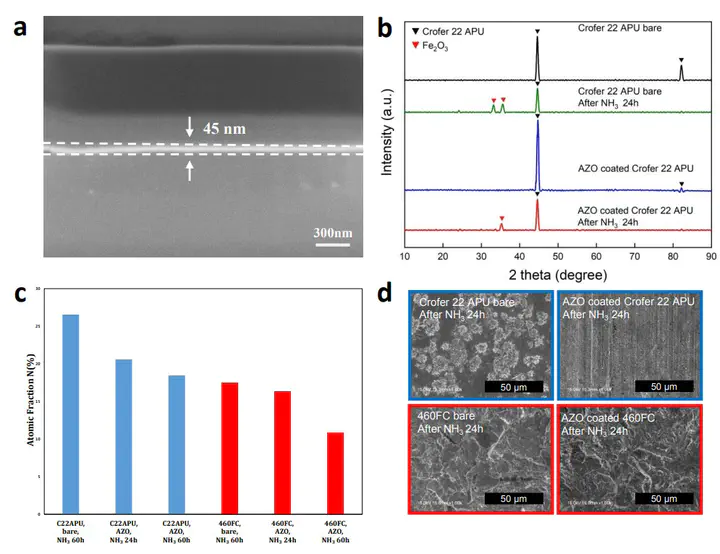
NH3 fuel-based SOFCs can overcome the inefficiencies of other hydrogen sources, but NH3 deteriorates solid oxide fuel cell (SOFC) separators by nitriding. Here, experimental and computational approaches are used to investigate aluminum-doped zinc oxide (AZO) as a SOFC separator coating candidate for nitriding mitigation. Experimental characterization reveals how AZO coating slows down the nitriding of steel separators, and that AZO conductivity improves upon NH3 exposure. A phase diagram construction framework combines a machine learning potential (MLP), density functional theory (DFT), and grand potential energy calculations to confirm the phase stability of AZO-based coatings under operating conditions. An a priori linear response simulation approach is developed to precisely reproduce ZnO electronic structure and conductivity. This methodology is adapted to explain why AZO-based coating color changes upon nitriding, the physical contributions to which are determined by AZO conductivity versus Al impurity concentration relationships, and explain how steam incorporation into AZO-based coatings can increase their conductivity. Energy-dispersive x-ray Spectroscopy (EDS), and the calculation of NHx adsorption energetics, infers ZnO surface nitriding susceptibility diverts nitrides away from other coating or separator components. This work demonstrates insights into the design of nitriding-resistant materials for NH3-based SOFCs.