Enhanced Hydrogen Evolution Reaction Performance of Ni-Doped MoS2 with 1T Structure for Alkaline Water Electrolyzer: Introduction of 1T Phase and Morphological Optimization Through Co-Sputtering Technique
Abstract
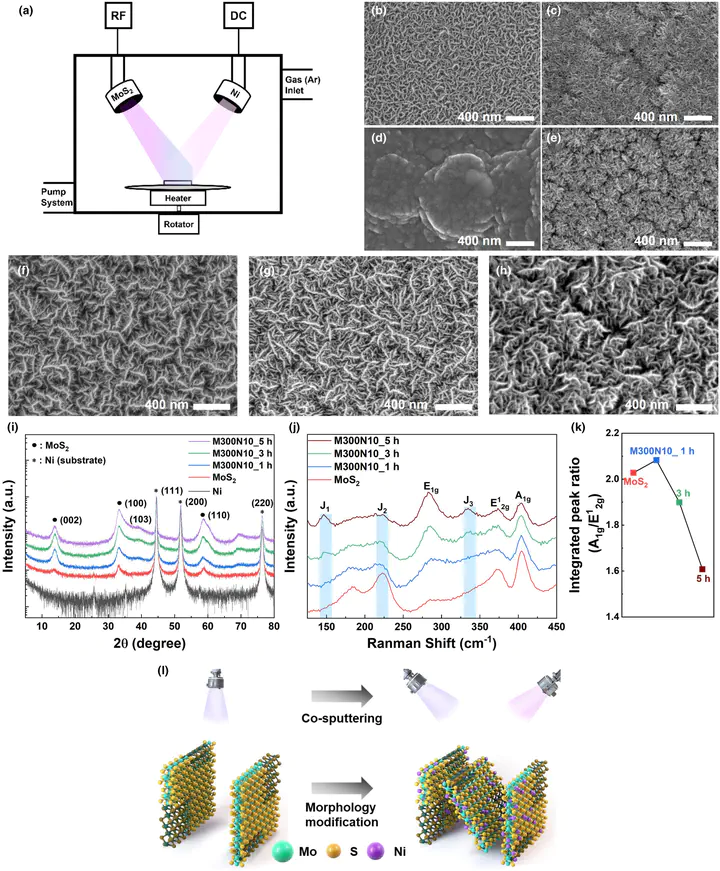
Molybdenum disulfide holds promise as a low cost and abundant catalyst for the hydrogen evolution reaction in an alkaline environment. However, its hydrogen evolution reaction activity is not sufficient for practical application because of its semiconducting properties in the 2H phase, presence of an electrochemically inert basal plane, and suboptimal hydrogen adsorption energy for hydrogen evolution reaction. In this article, we present a facile synthesis method for fabricating a Ni-doped molybdenum disulfide hydrogen evolution reaction electrode with a 1T structure through co-sputtering of molybdenum disulfide and Ni. Our results demonstrate that Ni doping not only promotes the 1T-phase yield in molybdenum disulfide structure but also activates the basal plane and improves the hydrogen adsorption energy of the edge plane. Also, the surface morphologies and 1T-phase yield, which are influenced by sputtering power and deposition time, are critical factors for the variation of hydrogen evolution reaction performance. Our Ni-doped molybdenum disulfide electrode, which exhibits high 1T yield and increased electrochemical surface area by tuning the morphology, shows an overpotential of ~91 mV at 10 mA cm−2, nearly 2.5 times lower than that of ~227 mV observed for molybdenum disulfide. Also, the single-cell test exhibits enhanced cell performance with improved durability in the repetitive on/off evaluation for the potential application of renewable energy integration.