Electrolyte-Engineered Photoelectrochemical Ammonia Oxidation Enabling Sustainable Hydrogen Production via Catalyst Regeneration
Abstract
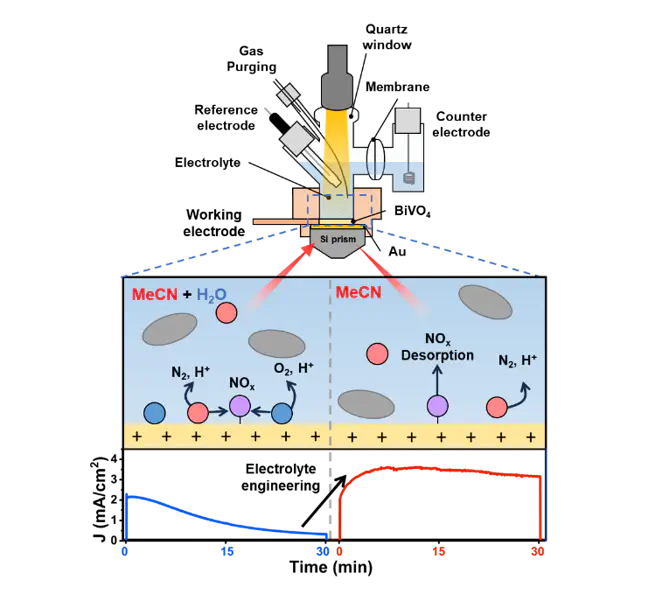
Ammonia oxidation reaction (AOR) offers a promising carbon-free hydrogen production pathway under ambient conditions, yet practical implementation faces critical challenges from catalyst deactivation and competing side reactions in aqueous systems. We present an electrolyte-engineered approach to photoelectrochemical (PEC) AOR that enables both enhanced hydrogen production and reversible catalyst regeneration. By employing a non-aqueous acetonitrile electrolyte at the BiVO4 photoanode, we suppress competing oxygen evolution and NOx poisoning, achieving a 6.9-fold higher hydrogen yield than aqueous systems. Spectroscopic and electrochemical analyses reveal that catalyst deactivation in water is not permanent but dynamically reversible upon re-exposure to non-aqueous environment, emphasizing the solvent-governed interfacial behavior. This electrolyte-engineering approach proves broadly applicable across metal oxide photoanodes (BiVO4, WO3, α-Fe2O3), establishing a universal design principle for PEC AOR systems. Our findings redefine the role of electrolyte composition in governing interfacial pathways and provide a practical framework for developing high-efficiency ammonia-to-hydrogen conversion platforms with enhanced durability and flexibility.