Generalized first-principles prediction of hydrogen para- equilibrium thermodynamics in metal hydrides
Abstract
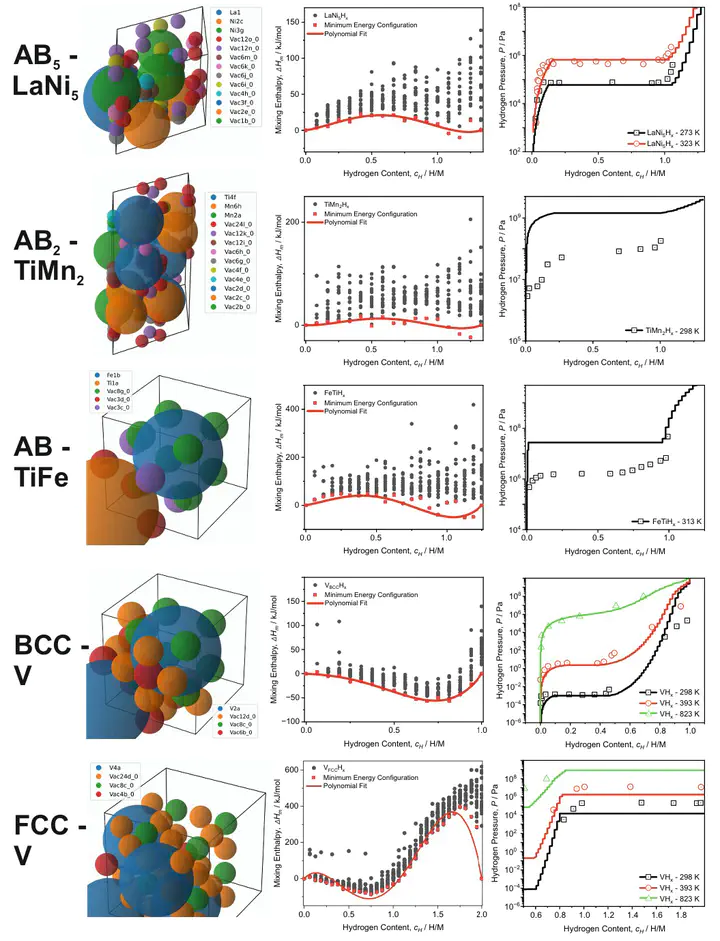
Metal hydrides are promising materials for storing, compressing, and purifying hydrogen by reversibly absorbing and releasing it. Accurate first-principles based prediction of the pressure–composition–temperature (PCT) relationships of metal hydrides can enable predictive optimization of hydrogen capacity and sorption pressures. In this work, we introduce a novel computational framework that integrates density functional theory (DFT) with a Python-based PCT Simulation Toolkit to predict PCT diagrams with high accuracy. By using only structural input data from the metallic phase, this toolkit automates the detection of interstitial voids, generates input files for DFT calculations, and constructs thermodynamic models based on para-equilibrium principles. We validate this approach across four major metal hydride classes– BCC alloys, AB5, AB2, and AB compounds– and demonstrate that even with minimal computational effort, key hydrogen sorption characteristics can be reliably determined. To evaluate the influence of exchange and correlation, we tested three different functionals– PBE, PW91, and r2SCAN– while applying the quasi-harmonic approximation to incorporate vibrational free energy contributions. Our results show that hydrogen capacity predictions achieve a mean accuracy of 95%, while sorption pressures are modeled within one order of magnitude of experimental values. Furthermore, we successfully extend this methodology to the BCC NbTiV multicomponent system, demonstrating its capability to construct comprehensive thermodynamic databases. This framework enables rapid and accurate exploration of known metal hydrides to design optimized alloys for new applications. Additionally, it serves as a predictive tool for designing novel hydrogen storage materials.