Microkinetic modeling of aqueous phase biomass conversion: Application to ethylene glycol reforming
Abstract
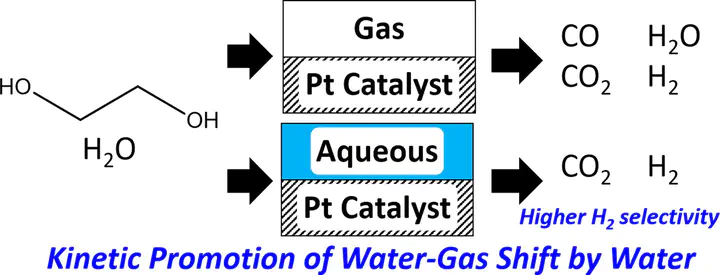
In this communication, we develop automatically a reaction network for ethylene glycol reforming on Pt. We employ a recently introduced group additivity scheme for estimation of thermochemistry over metal catalysts in vapor phase and in solution along with Brønsted-Evans-Polanyi (BEP) relations for vapor phase kinetics to parameterize microkinetic models for vapor and aqueous phases. Unlike vapor-phase reforming, we show that solvent occupies a significant fraction of surface sites and by doing this, it shifts the water-gas shift reaction to CO2 and H2 and avoids poisoning of the catalyst by CO. The model is found to be in reasonable agreement with the experimental data.
Type
Publication
Chemical Engineering Science, 2019, 197, 415-418